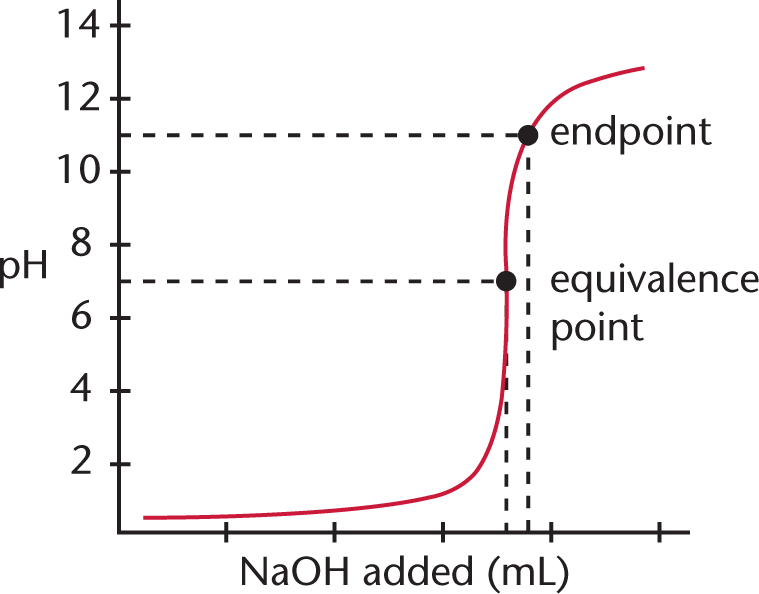
Titration Equivalence Point Endpoint . a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. You will also learn about equivalence points. endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the. suppose that a titration is performed and \(20.70 \: when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. \ce{naoh}\) is required to reach the end.
from schoolbag.info
The endpoint refers to the. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. You will also learn about equivalence points. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. endpoint and equivalence point are two terms commonly used in titration experiments.
Titration and Buffers Acids and Bases
Titration Equivalence Point Endpoint in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. The endpoint refers to the. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. \ce{naoh}\) is required to reach the end. endpoint and equivalence point are two terms commonly used in titration experiments. suppose that a titration is performed and \(20.70 \: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Titration Equivalence Point Endpoint endpoint and equivalence point are two terms commonly used in titration experiments. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. You will also learn about equivalence points. \ce{naoh}\) is required to reach the end. a titration is a volumetric technique in which a. Titration Equivalence Point Endpoint.
From www.pw.live
Difference Between Endpoint And Equivalence Point Titration Equivalence Point Endpoint the equivalence point of a chemical reaction is the point at which equal quantities of reactants. suppose that a titration is performed and \(20.70 \: You will also learn about equivalence points. \ce{naoh}\) is required to reach the end. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Equivalence Point Endpoint.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Equivalence Point Endpoint endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. in this tutorial,. Titration Equivalence Point Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Equivalence Point Endpoint suppose that a titration is performed and \(20.70 \: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. endpoint and equivalence point. Titration Equivalence Point Endpoint.
From www.numerade.com
SOLVED 3. Use a chemical dictionary or encyclopedia to explain the Titration Equivalence Point Endpoint You will also learn about equivalence points. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. suppose that a titration is performed and \(20.70 \: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. Titration Equivalence Point Endpoint.
From slideplayer.com
Ch. 15 & 16 Acids & Bases III. Titration (p ) ppt download Titration Equivalence Point Endpoint endpoint and equivalence point are two terms commonly used in titration experiments. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. suppose that a titration is performed and \(20.70 \: The endpoint refers to the. \ce{naoh}\) is required to reach the end. a titration is a volumetric technique in. Titration Equivalence Point Endpoint.
From sciencemotive.com
Differentiate Between Endpoint and Equivalence Point ScienceMotive Titration Equivalence Point Endpoint in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Titration Equivalence Point Endpoint.
From dokumen.tips
(PPT) TITRATION Titration of a strong acid with a strong base ENDPOINT Titration Equivalence Point Endpoint in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. suppose that a titration is performed and \(20.70 \: a point of equivalence. Titration Equivalence Point Endpoint.
From www.numerade.com
SOLVED How does the endpoint of titration differ from the equivalence Titration Equivalence Point Endpoint You will also learn about equivalence points. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. suppose that a titration is performed and. Titration Equivalence Point Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Equivalence Point Endpoint suppose that a titration is performed and \(20.70 \: You will also learn about equivalence points. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. when 25 ml of titrant has been added (the equivalence point), the ph is well above. Titration Equivalence Point Endpoint.
From www.slideserve.com
PPT Modern Chemistry Chapter 15 AcidBase Titration and pH PowerPoint Titration Equivalence Point Endpoint a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. in this tutorial, you will learn about titration. Titration Equivalence Point Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Equivalence Point Endpoint \ce{naoh}\) is required to reach the end. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. You will also learn about equivalence points. endpoint and. Titration Equivalence Point Endpoint.
From narodnatribuna.info
Ppt How To Interpret Titration Curves Powerpoint Titration Equivalence Point Endpoint when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points. endpoint and equivalence point are two terms commonly. Titration Equivalence Point Endpoint.
From mavink.com
Titration Endpoint Vs Equivalence Point Titration Equivalence Point Endpoint You will also learn about equivalence points. when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. \ce{naoh}\) is required to reach the end. a. Titration Equivalence Point Endpoint.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Titration Equivalence Point Endpoint when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. The endpoint refers to the. endpoint and equivalence point are two terms commonly used in titration experiments. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \:. Titration Equivalence Point Endpoint.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Titration Equivalence Point Endpoint when 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. The endpoint refers to the. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. You will also learn about equivalence points. a point of equivalence in. Titration Equivalence Point Endpoint.
From marielanewslee.blogspot.com
Explain the Difference Between End Point and Equivalence Point Titration Equivalence Point Endpoint \ce{naoh}\) is required to reach the end. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. a titration is a volumetric technique in which a solution. Titration Equivalence Point Endpoint.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Equivalence Point Endpoint You will also learn about equivalence points. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. suppose that a titration is performed and \(20.70 \: a point of equivalence in a titration refers to a point at which the added titrant. Titration Equivalence Point Endpoint.